Efficacy Data
Efficacy Data
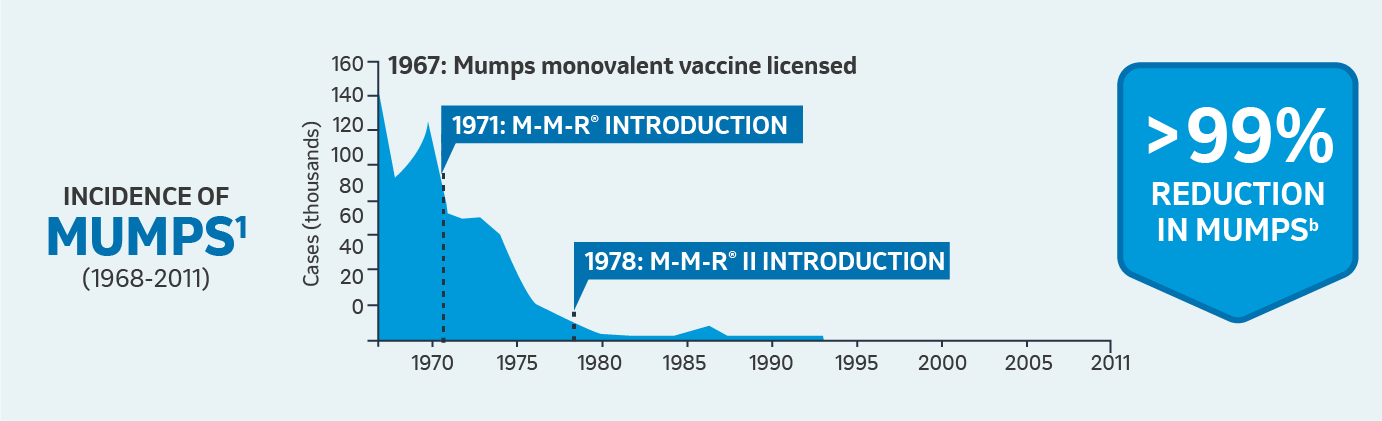
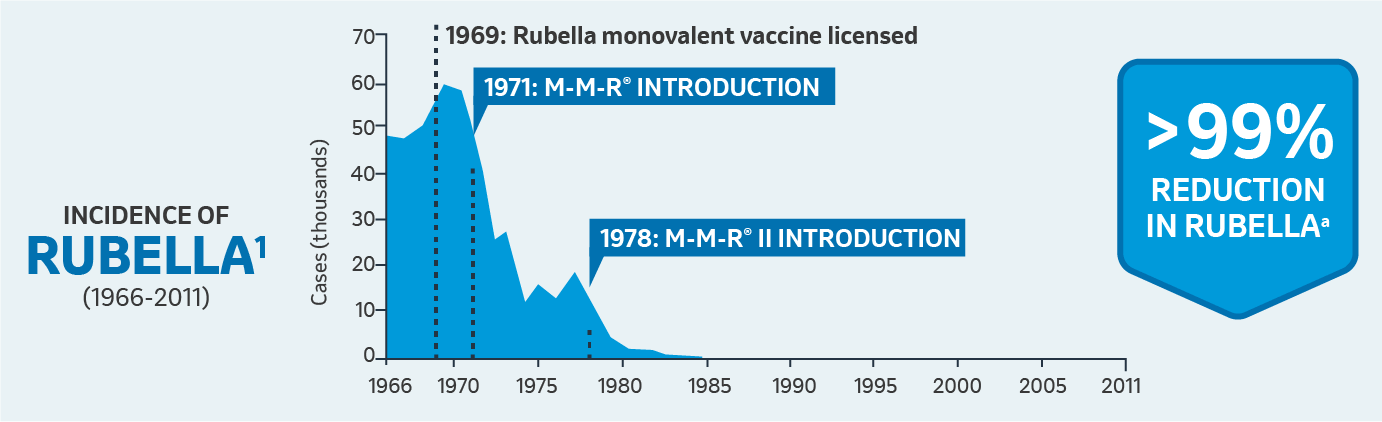
Safety

M-M-RII ® is well tolerated2
Consistent high performance during
- Routine use in multiple countries,
- In randomized controlled trials with diverse designs, and in outbreak settings, including use as measles postexposure prophylaxis
More about M-M-R®II
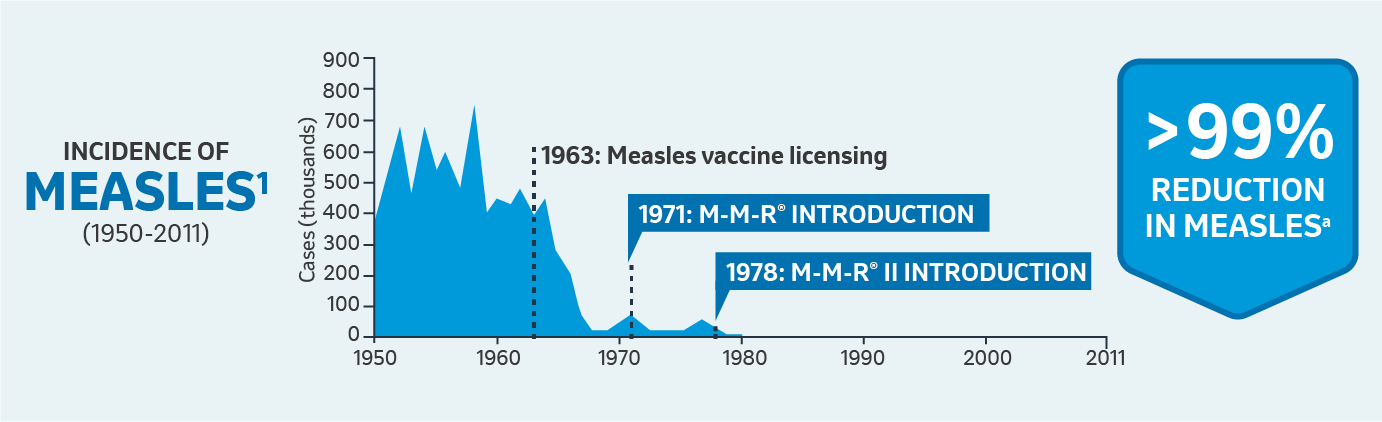
aFrom the year of the monovalent vaccine’s introduction to 2009.
bFrom the year after the monovalent vaccine’s introduction to 2009.
CDC surveillance of mumps cases began in 1968; CDC = Centers for Disease Control and Prevention.; MMR: Measles, Mumps, and Rubella
References:
- Kroger et al. Epidemiology and Prevention of Vaccine-Preventable Diseases. Centers for Disease Control and Prevention (CDC). 13th: The Pink Book. Washington DC. Public Health Foundation. 2015.
- Kuter BJ, Marshall GS, Fergie J, Schmidt E, Pawaskar M. Prevention of measles, mumps and rubella: 40 years of global experience with M-M-RII. Hum Vaccin Immunother. 2021 Dec 2;17(12):5372-5383. doi: 10.1080/21645515.2021.2007710.
PH-MMR-00015 Oct/2024